Ammonia in Aquaponics
Ammonia is an important player in any aquaponic system. Being one of the primary fish products and an engine of your system’s ecology, ammonia is important to understand. Hopefully this post will help you understand ammonia and ammonium.
What is ammonia?
Ammonia is a colorless gas that you probably know by its distinct, pungent smell. In an aquaponics system, this nitrogen/hydrogen compound is produced primarily by fish. The gas is toxic in high levels but necessary to give plants in a system the proper nutrients they need.
So how do we supply ammonia in non-toxic amounts?
For the most part, you can let nature have it’s way. Bacteria involved in the nitrogen cycle break down nitrogen compounds (including ammonia) into plant-available compounds like nitrates. This whole process starts when fish eat and digest protein, turning it into simpler nitrogen compounds. The end product of this step is ammonium, NH4+.
Difference between Ammonia and Ammonium
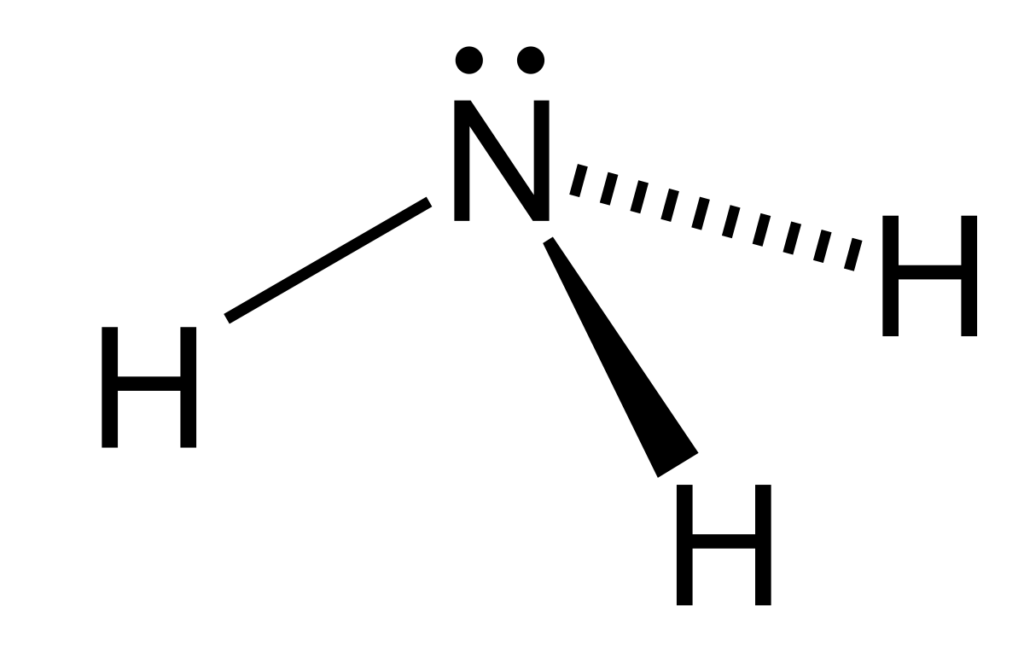
Ammonia: NH3
- Ammonia is more toxic than ammonium.
- Ammonia is a soluble gas.
- It has no charge, meaning that cells within the fish cannot regulate it.
Ammonium: NH4+
- Ammonium is positively charged.
- Ammonium is a byproduct of the metabolism of animals. Fish excrete it directly into the water.
- Due to the positive charge, fish are able to regulate the substance in and out of their cell membranes.
Ways that Ammonium enters the System
Ammonium gas, although toxic, is inherently part of the aquaponics system. There are several ways ammonium enters a system:
- Through the gills of a fish
- Excretion from the fish
Either or both of these ways may bring this necessary “nutrient” into play.
Aquaponic systems require nutrients to help the plants grow, and ammonium provides adequate nitrogen to plants.
pH and Ammonia/Ammonium Levels
To understand ammonia levels you must first understand pH.
- The higher the pH in a system, the higher the level of ammonia and subsequently the lower the level of ammonium.
- The lower the pH in a system, the lower the level of ammonia and subsequently the higher the level of ammonium.
In your system you want to aim for a relatively low level of pH, making ammonium the dominant substance in the system.
Nitrification efficiency is a problem many people run into. A higher level of pH increases the oxidation efficiency in a new system. Generally, folks will gravitate toward having higher pH in their system. However, as the system gets older it adapts, and efficiency can be reached at lower pH values.
We suggest a pH level of 6-6.4.
How to Reduce Ammonia Levels
1. Reduce the amount of nitrogen going into your system
You can bring down ammonia by avoiding overfeeding. If you overfeed, ammonia levels will jump up. To avoid this, cut back on feeding those fish. You also should watch out for dead fish, which secrete ammonia. If you find a dead fish, remove it, then do not feed that tank for a day. (Rotting feed will have the same affect as a dead fish.)
2. Increase Nitrification Efficiency
This method increases the rate at which your ammonia or ammonium is being oxidized, speeding up the conversion of them into nitrates, which plants can pick up. Try to have nitrates in your system. They are nontoxic to fish.