PH is the measure of a solution’s acidity. It ranges from 0 – 14; at 25C anything less than 7 is acidic whereas anything above 7 is basic. This neutral point drops as the temperature increases, which is why it’s important that pH sensors compensate for changes in temperature.
The normal range for most hydroponic crops is between 5.5 and 6.5: slightly on the acidic side of neutral. There are definitely outliers so it’s important to research which varieties prefer which pH levels and grow them accordingly. Most hydroponic crops will tolerate a range in pH and you can settle on a compromise that suits the majority of your plants even if none are strictly optimized; certain crops won’t make good neighbours, however, as their pH range does not overlap.
How PH works
PH is sometimes referred to as the ‘Power of Hydrogen,’ because it’s a measure of the free hydrogen ions (H+) versus the hydroxyl ions (OH-) in a given solution. The more hydrogen ions, in relation to hydroxyl ions, the more acidic a solution is and, conversely, the more hydroxyl ions are present, the more basic a solution is. This means that a solution with pH 7 has an exact balance between positively charged hydrogen ions and negatively charged hydroxyl ions.
The PH scale is logarithmic. This is a mathematical term that tells us how to decode the strength of one acid against another. it breaks down like this: every full step along the scale represents 10X more potency. So if we start at pH 6 and go down to pH 5 there are 10X more free hydrogen ions (H+) in solution and therefore 10X stronger acidity. The same works on the other side: going from pH 8 to pH 9 there are 10X more hydroxyl ions (OH-) making the latter solution 10X more basic (or less acidic, if you prefer).
Why PH matters?
Normally when we think of acids and bases what comes to mind is extreme examples; things like vinegar, battery acid, and bleach. While strong bases and acids have a place in hydroponics (which we’ll address below), we’re interested here in the subtle changes in water chemistry that allows for nutrient uptake and therefore healthy plant growth. When we say that our nutrient solution is too acidic or basic it’s often a question of being only a few decimal points outside of our desired range; rarely is the water so acidic or basic that we need to fear root damage (though this can happen as well, if something is not balanced).
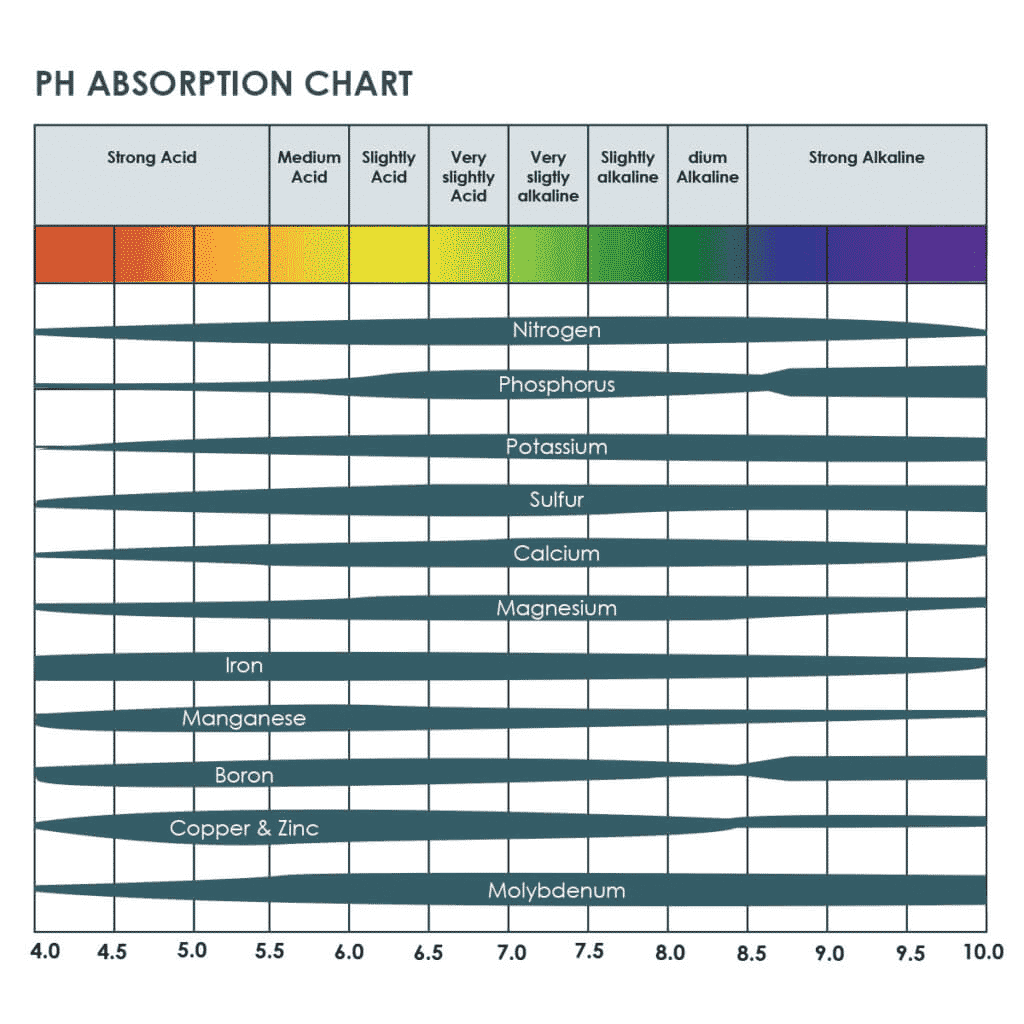
For most crops the desired range is right around 6–just on the acidic side of neutral. This is a convenient window where all of the nutrients required for healthy plant growth are available for uptake. In general, as solution becomes more acidic macronutrients become less available, and conversely, with a rise in PH micronutrients become locked out. It quickly becomes apparent why we need to constantly monitor our solution’s PH. Often it’ll happen that all the nutrients have been dosed in the appropriate ratios and amounts but still the crop suffers from some deficiency due to PH being out of range… The nutrients are right there, but the plants aren’t able to make use of them. This is always one of the first things to check if you’re noticing nutrient deficiencies. Many novice farmers will immediately dose extra nutrients the moment they’ve identified a deficiency thus working against themselves.
Balancing PH
The PH of your solution is going to change as nutrients are added. Normally nutrients acidify the solution, which is why balancing PH is done after the desired nutrient level is achieved. Once nutrients have been dosed appropriately then we turn to balancing the PH; in hydroponics this is commonly done using very small amounts of strong acids (sulfuric, citric) or bases (potassium hydroxide, potassium carbonate) mixed into the reservoir and checked repeatedly to avoid overdosing.
Most systems should trend in one direction only. This means that without active PH adjustment the nutrient solution will either gradually increase or decrease, but not both. There is a natural fluctuation in PH over time as plants take certain nutrients out of solution, and as different elements interact with another, but over all the solution will drift either up or down. Automated dosing can make this task much easier as the farmer need only identify the trending direction of PH in their system and install a source of either PH up or PH down to be dosed as needed.
Be aware, when using an automated dosing system to have only the appropriate PH modifier installed. If your system is set up with both PH up and PH down and is programmed to maintain a narrow window it can cause problems. The doser will be working against itself if the desired PH range is too narrow which will result in wasted resources and poor water chemistry.